Role of non-canonical post-translational modifications in gastrointestinal tumors
Post-translational modifications (PTMs) of proteins contribute to the occurrence and development of tumors. Previous studies have suggested that canonical PTMs such as ubiquitination, glycosylation, and phosphorylation are closely implicated in different aspects of gastrointestinal tumors. Recently, emerging evidence showed that non-canonical PTMs play an essential role in the carcinogenesis, metastasis and treatment of gastrointestinal tumors. Therefore, we summarized recent advances in sumoylation, neddylation, isoprenylation, succinylation and other non-canonical PTMs in gastrointestinal tumors, which comprehensively describe the mechanisms and functions of non-classical PTMs in gastrointestinal tumors. It is anticipated that targeting specific PTMs could benefit the treatment as well as improve the prognosis of gastrointestinal tumors.
Introduction
Protein post-translational modifications (PTMs) are biochemical mechanisms by which amino acid residues in proteins are covalently modified. Until now, over 400 kinds of PTMs of proteins have been identified, of which canonical PTMs include acetylation, ubiquitination, phosphorylation, methylation and so on. Through adding small chemical groups or small proteins, inactive precursor proteins change into functional mature proteins of diverse properties. Emerging evidence suggested that PTMs of proteins participate in tumorigenesis, progression, prognosis and treatment of cancers [1,2,3]. In recent years, multiple non-canonical protein PTMs were identified including SUMOylation, isoprenylation and palmitoylation, which have also been found to be involved in various aspects of tumors.
Gastrointestinal tumors continue to be one of the most common tumors worldwide and pose a significant global public health burden, with their high rates of incidence and mortality. A variety of factors such as genetics, diet, infection, and epigenetic changes give rise to the carcinogenesis and development of gastrointestinal tumors [4, 5]. Recent investigations indicate significant involvement of non-canonical protein PTMs in gastrointestinal tumors. For example, SUMO modification of annexin A4 (ANXA4), a calcium-binding protein located in the gastrointestinal tract, increases its expression and promotes epithelial-mesenchymal transition (EMT) of tumor cells [6]. In human colorectal tissues, the K420 site lysine crotonylation of alpha-enolase (ENO1) enhances its enzyme activity, thus promoting tumorigenesis and aggressiveness [7]. NEDD8-Activating Enzyme inhibitor MLN4924 has been reported to suppress the growth and migration of gastric cancer cells by blocking the neddylation of the E3 ubiquitin ligase CRL [8]. In addition, CPT1A promotes lysine residue k222 succinylation of lactate dehydrogenase A (LDHA), which is associated with tumor cell proliferation and poor prognosis of patients [9].
In this review, we summarized recent advances in the studies investigating the role of non-canonical PTMs including SUMOylation, isoprenylation, neddylation, citrullination, succinylation and other modifications in the occurrence, development, treatment and prognosis of gastrointestinal tumors. It is anticipated that understanding and targeting these novel protein PTMs could benefit therapeutic strategies for gastrointestinal tumors.
SUMOylation
SUMOylation is a dynamic and reversible post-translational modification mediated by E3 ligases, dimeric SUMO E1 SAE1/UBA2 and a single E2 ubc9, often with the involvement of protein inhibitor of activated STAT (PIAS) family members, and Ran binding protein 2 (RanBP2) and SUMO protease SENPs (Sentrin-specific proteases). Small ubiquitin-like modifier (SUMO) is a highly conserved 11 KDa protein, of which four isoforms of SUMO are known, including SUMO1, SUMO2/3 (SUMO2 and SUMO3 have 95% sequence homology) and SUMO4. SUMOylation is involved in multiple biological processes such as genomic stability, cell differentiation, DNA damage response, cancer cell proliferation and invasion [10, 11] (Fig. 1).
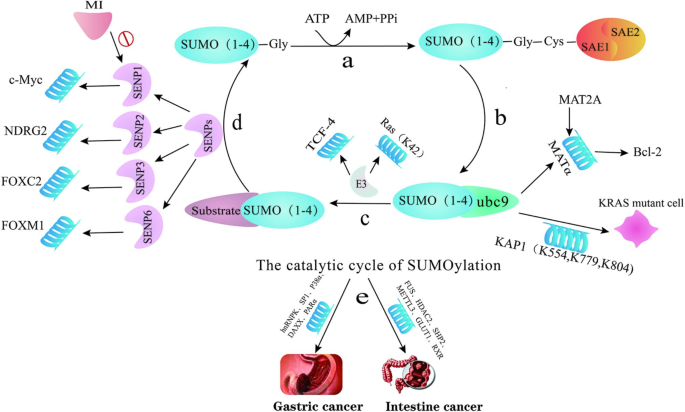
SENP-mediated SUMOylation
SENPs are SUMO-specific proteases, originally known as Sentrin-specific proteases, that remove SUMO from target proteins. There are six members of the mammalian SENP family, which can be bifurcated into three subfamilies, SENP1 and SENP2 as one family, SENP3 and SENP5 as one family, and the remaining SENP6 and SENP7 as one family [12, 13].
It was found that SENP1 was overexpressed in colon cancer tissues, and silencing SENP1 expression could inhibit the growth of colon cancer cells by arresting the cells in G1 phase [14]. Momordin Ic(MI), an emerging SENP1 inhibitor, can act as an anti-cancer drug by inhibiting the de-SUMOylation of c-Myc and reducing the level of c-Myc protein, which lead to cell cycle arrest and apoptosis of tumor cells [15]. In addition, SENP2 can mediate the de-SUMOylation and stabilization of N-myc downstream regulatory gene 2 (NDRG2) in gastric cancer, thereby exerting its anti-tumor effect [16].
The transcription factor forkhead box proteins play an essential role in regulating the cell cycle and promoting the development of gastric cancer. SNEP3 activates the transcriptional activity of forkhead box protein C2 (FOXC2) and increases the expression of N-cadherin through the deSUMOylation of FOXC2, which activates the transformation of epithelial mesenchyme as well as enhances the invasion and metastasis of gastric cancer cells [17]. The transcription factor forkhead box protein M1 (FOXM1) has been suggested to promote tumor formation by regulating cell cycle. SENP6 activates and stabilizes FOXM1 by catalyzing the de-SUMOylation of FOXM1, which leads to successful mitosis and promotes the growth of gastric cancer cells [18].
Non-coding RNAs-regulated SUMOylation
LncRNAs refer to non-coding RNA with a length of more than 200 nucleotides [19]. CircRNAs are non-coding RNA with ring stricture, which stably express and regulate multiple biological processes [20]. Both lncRNAs and circRNAs have been found to regulate SUMOylation in gastrointestinal tumors.
In gastric cancer, LncRNA SDCBP2-AS1 blocks the SUMOylation of hnRNPK (heterogeneous nuclear ribonucleoprotein in K) via binding to the KH domain of hnRNPK, which destroys the stability of β-catenin and promotes its degradation, thus inhibiting the growth and migration of gastric cancer cells [21]. SUMOylation inhibition of SP1 increases its expression level, which promotes the expression of LncRNA SNHG17 and alters the miR-23b-3p/Notch2 pathway, thus promoting the occurrence and development of gastric cancer [22]. Besides, SUMO1 pseudogene 3 (SUMO1P3), a member of the SUMO pseudogene family that expresses lncRNA, is up-regulated in gastric cancer, which correlated with tumor size, differentiation, metastasis and invasion [23].
GAL (GLUT1 associated LncRNAs) is the lncRNA associated with colorectal liver metastasis (CRLM). In CRLM tissues, the expression of GAL is up-regulated to promote SUMOylation of GLUT1 protein, thus promoting metastasis of colorectal cancer by preventing the degradation and enhancing the stability of GLUT1 [24]. RMST, a proliferation and apoptosis-related lncRNA, binds with FUS located in the nucleus and enhances SUMO1 modification at the K333 site of FUS, thereby enhancing the stability of the complex, which promote mitosis and migration of colon cancer cells [25]. In addition, circ-GALNT16 can inhibit SENP2-mediated hnRNPK de-SUMOylation by binding to the KH3 domain of hnRNPK and inhibit the progression of colorectal cancer [26].
Drug resistance-related SUMOylation
At present, the major treatment of gastrointestinal tumors is surgical treatment supplemented by chemoradiotherapy. For patients in advanced stages, radiotherapy and chemotherapy remain the main treatments. However, many drugs are prone to drug resistance in clinical practice [27]. Recent studies demonstrated that SUMOylation contributes to drug resistance in gastrointestinal tumors.
The expression of methyltransferase-like 3 (METTL3) is increased through SUMO1 SUMOylation modification in colorectal cancer, and increased METTL3 promotes the expression of ABCC1 protein, thus leading to drug resistance of colorectal cancer cells to chemotherapy [28]. The SUMOylation of IGF1R allows nuclear translocation of the transcriptional cofactor IGF1R, which is associated with drug resistance in colorectal cancer [29]. In addition, SUMOyaltion of deacetylase 2 (HDAC2) can protect colon cancer cells from chemotherapeutic-induced genotoxic stress by activating kinase RSK1 and enhancing the regulatory transcription of nuclear factor-KB (NF-KB), so high levels of HDAC2 correlated with chemotherapeutic-drug resistance in colon cancer [30].
Topoisomerase I (Popo-I) inhibitor irinotecan (CPT-11) and cisplatin (DDP) are commonly used in the treatment of gastrointestinal tumors [31]. In drug-resistant cells of colorectal cancer, the aberrantly expressed SENP1 could increase the SUMOyaltion of HIF-α, which leads to the conversion of intracellular signal transduction pathway from Wnt/β-catenin to EGFR/IKKα/β/NF-kB, thus reducing the sensitivity of cancer cells to irinotecan [32]. Furthermore, in cisplatin-resistant gastric cancer tissues, it was found that low-SUMOylation of SP1 increased the expression of SP1 in gastric cancer tissues, and the increased SP1 promoted drug resistance of gastric cancer cells through the SNHG17/miR-23b-3p/Notch2 axis [22].
Oxidative stress and adaptation to hypoxia
Various intracellular and extracellular stimuli are involved in the pathophysiological processes of different diseases, of which oxidative signal is closely correlated with the occurrence and development of various tumors [33].
An anoxic microenvironment is a challenge that tumors must adapt to and overcome in order to survive [34], and in the anoxic environment, gastric cancer cells adapt to anoxic conditions by upregulating lysine-specific demethylase 5B (KDM5B). By enhancing the SUMOylation of KDM5B mediated by SUMO3 and responsible for SUMO E3 ligase PIAS4, hypoxia promotes the stability of KDM5B, which is conducive to the response of tumor cells to anoxic environment [35]. In addition, ROS promoted SUMO1-mediated p38α SUMOylation at the K152 site by stabilizing E3 SUMO protein transferase PIASxα, which ensures the stability and nuclear translocation of p38α as well as promotes the metastasis of gastric cancer cells [36]. SENP3 has been reported as a specific protease sensitive to redox reactions in gastrointestinal tumors [37]. SENP3 enhances the transcriptional activity of FOXC2 by participating in de-SUMOylation of FOXC1 under oxidative stress, thereby promoting EMT [17]. In colorectal cancer, the SUMOylation of PIAS itself can also regulate the function and stability of the protein. PIAS promotes HIF-1’s SUMOylation-mediated hypoxia adaptation by interacting with the ODD domain of HIF-1 [35, 38].
ubc9
Ubiquitin-coupled enzyme 9 (ubc9), an essential E2-coupled enzyme of SUMOylation, has been found to prolong tumor cell survival through the function of B-cell lymphoma 2 (Bcl-2) in diverse cancers [39].
Methionine adenosine transferase 2 A (MAT2A) encodes MATα2, which is highly expressed in colon cancer and regulated by the SUMOylation of SUMO1, SUMO2 and SUMO3 [40, 41]. MATα2 could interact with ubc9 to increase Bcl-2 protein [42]. It was found that the deficiency of ubc9 in mice promoted cell growth and the formation of polyps. In colon cancer, increased ubc9 suppressed effective SUMOylation and promoted tumorigenesis [43].
RAS, a signal transduction molecule downstream of growth factor receptors, regulates the biological function of cells through multiple pathways and is often accompanied by KRAS gene mutation in colorectal cancer [44]. The SUMO ligase ubc9 is necessary for KRAS, and the activity of transcriptional repressor KAP1 is enhanced in KRAS mutated cells after SUMOylation at K554, K779, and K804 [45].
Cell cycle and cell proliferation
The occurrence and development of gastrointestinal tumors are complex processes with various aspects involved, one of which is the excessive proliferation of cells [46]. SUMOylation can regulate tumor cell cycle and thus regulate tumor cell proliferation.
When carcinogenic stress signals in the body respond, the scaffold protein IQCAP1 regulates alternative splicing(AS) related to the cell cycle of gastric cancer cells by influencing the SUMOylation of the AS regulatory protein hnRNPM, thus promoting the proliferation of tumor cells [47]. Moreover, in colorectal cancer, IQGAP1 promotes the proliferation and migration of tumor cells by promoting the phosphorylation of ERK, MEK, and AKT through SUMO1-mediated SUMOylation at the lysine K1445 [48].
V-maf musculoaponeurotic fibrosarcoma oncogene family protein B(MAFB), a member of the Maf transcription factor family [49], plays a critical role in colorectal cancer. Mediated by SUMO1, MAFB is SUMOylated at the lysine 32 site, which affects its binding to cell cycle factors such as CDK6 and regulates G1/S phase transition [50]. Thus, MAFB plays a crucial role in promoting tumor cell proliferation and inhibition of MAFB leads to cell cycle arrest. Momordin Ic (MI) demonstrated therapeutic potential by inhibiting the SENP1/c-MYC signaling pathway allowing colorectal cell cycle arrest in the G0/1 phase [15].
Inhibition of SUMO1-activating enzyme subunit 1 (SAE1) can block colon cancer cells in the G0/G1 phase [51]. At the same time, ubiquitin-like modifier activating enzyme 2 (UBA2), namely SUMO activating enzyme subunit 2 (SAE2), is a crucial component of SUMOylation E1 enzyme [52]. Although the specific mechanism of UBA2 and SUMOylation remains unclear, inhibition of de-SUMOylation activation of UBA2 can reduce the levels of cyclingB1, Bcl-2, DMD2, p-AKT and affect p53/DMD2/p21 signaling pathway, thus influencing the transitions of the colorectal cancer cell cycle from G1/S and G2/M [53]. In colorectal cancer, high UBA2 expression is positively correlated with EZH2 expression and unfavorable prognosis of patients. E2F1 can be modified by SUMO1 at Lys-226, and SUMOized E2F1 promotes the expression of EZH2 by enhancing its binding to the EZH2 promoter [54].
CSC
Cancer stem cells (CSCs), also known as tumor-initiating cells, are one of the main causes of colorectal cancer recurrence and metastasis as well as poor therapeutic effect during radiotherapy and chemotherapy [55].
Many transcription factors, including the AP-2 transcription factor, regulate the EMT process and are regulated by SUMOylation [56]. The SUMOylation of TFAP2A (transcription factor activating protein 2α) occurs at lysine 10 [57], and the binding of TFAP2A to SUMO is inhibited by E1 and E3 SUMO inhibitors. The CSCs of colorectal cancer are CD44 +/hi ALDH +/hi cell population which are mediated by SUMO-unconjugated TFAP2A. SUMO inhibitors inhibit colorectal cancer growth, invasion, and metastasis by inhibiting the CSCs population through the inhibition of CD44 and MMP14 [58].
ALDH, which is highly expressed in colorectal cancer CSCs, is not only a marker but also important for biological functions such as maintenance and self-renewal of CSCs [59]. TRIM21 acts as the ubiquitin E3 ligase of Oct-1, and the SUMOylation enhances the ubiquitination and degradation of Oct-1 (ALDH transcription factor) and reduces its stability by regulating the expression of its transcription factor interferon regulatory factor 1 (IRF1) [60].
TCF-4 and NPC
T cell factor 4 (TCF-4) can bind to nuclear pore complex (NPC) protein, which is associated with the Wnt/β-catenin signaling pathway involved in tumorigenesis of colorectal cancer. The SUMOylation of TCF-4 promotes its interaction with β-catenin, while RanBP2, an NPC protein that acts as SUMO E3 ligase, can regulate the SUMOylation of TCF-4 by using RanGAP1 as the substrate and mediated by SUMO1. In addition, the overexpression of NPC protein can promote TCF-4 transcriptional activity and the Wnt/β-catenin signaling pathway [61]. It has also been found that SUMOylation mediated by UHRF2 regulates the stability of TCF-4 protein, thereby maintaining the stability of the Wnt/β-catenin signaling pathway, which in turn regulates UHRF2 expression in CRC [62].
The accumulation of death domain-associated protein (DAXX) in the nucleus of tumors is associated with the poor prognosis of gastric cancer. As a substrate of SUMO2/3, DAXX modulates its level by binding to SUMO2/3, and it regulates the cytoplasmic distribution of DAXX and increases the stability of the protein by affecting the SUMOylation, which is catalyzed by RanBP2/RanGAP1 [63]. Ras protein, another common NPC protein, is subject to SUMO3-mediated SUMOylation at lysine K42 and influences the occurrence and migration of colorectal cancer via the Raf/MEK/ERK signaling axis [64].
Others
A number of other researches have been conducted on the relationship between SUMOylation and gastrointestinal tumors. P53 is the modified substrate of SUMO1, and SUMO1-mediated SUMOylation leads to increased levels of the p53 protein in colon cancer patients, which is associated with poor prognosis of patients [65]. In colorectal cancer, the zinc-finger-containing transcription factor KLF5 promotes nuclear localization and regulates cell proliferation through SUMOylation of glutamic acid residues at positions 153 and 204, and stabilizing KLF5 in the nucleus by inhibiting its leucine-rich nuclear exit signaling (NSE) activity [66]. CBX4 rs77447679 gene polymorphism was positively correlated with the high risk of gastric cancer, while the CC genotype had a low risk of gastric cancer [67]. In CRC, anti-PD-L1 antibody combined with irradiation (IR) + ATR inhibitor (ATRi) can enhance the efficacy of the therapy, which is based on the mechanism of activating STRING signaling by promoting SUMOylation of SHP1 at the lysine 127 locus, thus inducing the expression of IFN-I-related genes and promoting immunotherapy [68].
In patients with gastric cancer, the high expression of TRIM28 is closely related to the low survival rate of patients, which may be related to the direct binding and stabilization of PD-L1 by inhibiting the ubiquitination of checkpoint protein PD-L1 in gastric cancer cells and promoting its SUMOylation [69].In gastric cancer cells, it was found that all-trans retinoic acid (ATRA) could enhance the SUMOylation of retinoic acid receptor α (RARα) and improve its stability, thus promoting the formation of RARα and PXRα heterodimers in the nucleus, which is favorable to its signal transduction [70]. NSUN1-7 is a nucleolar RNA methyltransferase highly expressed in gastric cancer and plays a vital role as a writer for RNA modification of 5-methylcytosine (m5C) [71]. SUMO2/3-mediated SUMOylation of NSUN1-7 regulates its protein stability and promotes its transportation to the nucleus, thus exerting a carcinogenic role dependent on m5C methyltransferase [72]. Ginkgo acid (GA), a natural component of ginkgo, nuts, and seed coat [73], inhibits the migration, proliferation, and EMT of gastric cancer cells by inhibiting SUMO1-mediated SUMOylation of IGF-1 and reducing the expression level of SNA12 and its binding to IGF-1 [74].
Neddylation
Neddylation is a reversible post-translational modification of proteins catalyzed by NEDD8-activated enzyme E1 (NAE), Nedd8-coupled enzyme E2 (UBE2F, UBE2M) and substrate nedd8-E3 ligase, which plays a crucial role in various biological processes such as tumorigenesis, tumor microenvironment and apoptosis by binding the ubiquitin-like protein NEDD8 to lysine residues of substrate protein [75, 76].
CRLs and CUL
E3 ubiquitin ligases, generally classified into scaffold type and thioester bond intermediate type according to the difference between RING domain 1 and HECT domain 2 [77], play an important role in the biological functions of gastrointestinal tumors by participating in neddylation modification.
Under the mediation of NEDD8 E3 ligase Mdm2, neddylation of Hu antigen R (HuR) at K283, K313 and K326 promotes the stability of HuR protein and stabilizes its localization in the nucleus, which promotes the proliferation of gastrointestinal cancer cells [78]. The most abundant member of E3 ubiquitin ligases is the Cullin-RING E3 ligases (CRLs), a kind of crucial catalytic enzyme activated by the substrate cullin(CUL) during the neddylation. The CUL family has a total of seven members including CUL1, CUL2, CUL3, CUL4A, CUL4B, CUL5, and CUL7 [79]. CUL plays an important linker role in CRLs, and the neddylation of CUL and abnormal activation of CRLs are closely associated with gastrointestinal tumors (Table 1).
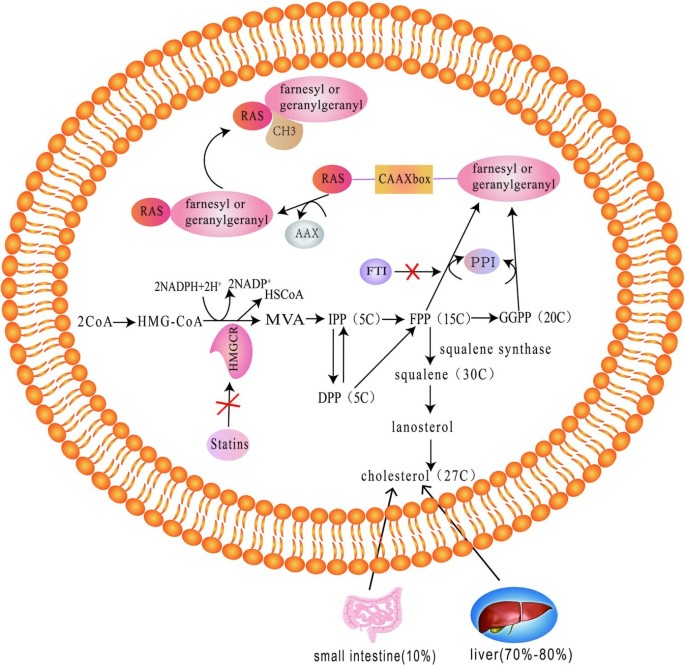
RAS proteins
The RAS oncogene family includes Kirsten RAS (KRAS), Harvey RAS (HRAS) and neuroblastoma RAS (NRAS), and ras oncogenic proteins include H-RAS, N-RAS, K-RAS4A and K-RAS4B, among which KRAS is the most widely studied [107]. The function of Ras protein depends on post-translational modification, which firstly requires farnesylation, that is, 15-carbon isopentyl farnesyl can be added to the cysteine sulfhydryl group of CAXX box at the carboxyl terminus of ras under the catalysis of farnesyl protein transferase. The protein then hydrolyzes AXX amino acids and methylates the carboxyl group of farnesylated cysteine [108].
Farnesyltransferase inhibitors (FTI) and geranylgeranylation inhibitor
Farnesyltransferase initiates the signaling of ras proteins attached to the plasma membrane [109]. FTI blocks the membrane localization of ras and its signaling pathway by inhibiting the isoprenylation of H-RAS rather than K-RAS, so H-RAS might be a therapeutic target for FTI-mediated reflex sensitization in patients with colorectal cancer [110, 111]. FTI exerts its anti-CRC effect by inhibiting the farnesylation of ras proteins and other peptides through complex mechanisms. On the one hand, FTI can act as an analogue of FPP, competitively inhibiting FPP binding to FTase and CAAX sequences of K-RAS; On the other hand, in the presence of FTI, the N- and K-RAS proteins of farnesyltransferase substrates may be geranylgeranylated [112, 113].
KT7595, a gliotoxin derivative, and the farnesyl transferase inhibitor manumycin derived from streptomyces can inhibit FPTase by inhibiting the farnesylation of the p21 and the activity of the p42MAPK/ERK2 in its downstream pathways, thus inducing apoptosis and inhibiting proliferation of gastrointestinal tumor cells. Notably, its mechanism does not involve the cholesterol synthesis pathway [114,115,116]. In addition, the inhibitor of geranylgeranylation, BAL9611, similar to manumycin, can inhibit geranylgeranylation of P21rhoA and then inhibit the phosphorylation of P42ERK2/MAPK, thereby inhibiting K-ras mutant colon cancer cells [117].
Statins
Statins, also known as HMG-CoA reductase inhibitors, work by inhibiting the mevalonate pathway by blocking the conversion of HMG-CoA to mevalonic acid, which is a rate-limiting step in cholesterol synthesis [118]. The final metabolites of the mevalonate pathway, farnesyl and geranyl, are substrates for the isoprenylation of Ras proteins [119]. The use of statins can reduce the risk of tumors, and multiple studies have found that statins may be associated with isoprenylation of Ras proteins, which modulates the pathway where Ras proteins are located and exert their therapeutic effects on tumors [120, 121].
In RAF-driven colorectal cancer, statins can inhibit the isoprenylation of Ras protein which is mediated by the downstream intermediates FPP and GGPP of the cholesterol synthesis pathway, and inhibit the activation of the PI3K/AKT pathway as well as Hippo pathway to inhibit the activity of cell growth [122].
KRAS protein is activated by isoprenylation, and activated Ras acts as the switch in EGFR and nucleus, which is critical for ras/raf/MAPK and PI3K/AKT pathways [113]. EGFR inhibitor cetuximab can be used as a superior chemotherapy drug for advanced colorectal cancer patients without KRAS mutation [123]. While for patients with KRAS-mutated metastatic colorectal cancer (mCRC) that abnormal activation of the RAS pathway leads to abnormal proliferation of tumor cells, statins may treat mCRC by inhibiting isoprenylation of ras. Notably, the use of statins such as simvastatin has not been found to enhance the efficacy of cetuximab in patients with mCRC [124, 125].
It was found that pravastatin inhibits the isoprenylation of growth-regulating proteins such as P21 ras to inhibit the synthesis of cholesterol [126, 127], however, it has been reported the efficacy of hydrophobic simvastatin is superior to hydrophilic pravastatin in the treatment of the majority of tumors including intestinal cancer, especially in the treatment of poorly differentiated cancer cells [128]. In addition, the apoptosis induced by lovastatin in human colon cancer cell line SW480 was also achieved by inhibiting the synthesis of cholesterol. The blocking of the isoprenylation of ras protein inhibits the activation of PI3 kinase, thus reducing the expression of anti-apoptotic molecule survivin and the apoptosis caused by the interference of survivin to RNA [129]. However, it has also been shown that lovastatin functions not by inhibiting farnesylation of the target protein, but by suppressing geranylgeranylation [130].
Other studies on ras proteins
PDEδ is a guanine nucleotide dissociation inhibitor (GDI-) -like solubilizing factor that can bind to the C-terminal farnesyl of KRAS to interfere with the localization and signaling pathways of ras, and influence the survival and proliferation of cells [131, 132]. Therefore PDEδ inhibitors might become a novel target for KRAS mutant colorectal cancer.
Curcumin extracted from the spice turmeric can take polyisoprenylated methylated protein methyl esterase (PMPMEase) as the target to reversibly inhibit the activity of PMPMEase, thus inducing the death of colorectal cancer cells [133]. Similar to statins, dehydroepiandrosterone (DHEA) inhibits the synthesis of cholesterol and the isoprenylation of P21 ras as well as its binding to cell membranes in colorectal cancer [134].
Monoterpenes, the non-nutritious dietary substances in citrus fruits and some plant essential oils, may exert antitumor effects by influencing the isoprenylation of other proteins except ras. For example, limonene, coriander oil and carvone can prevent patients from suffering from gastric cancer, and the mechanism may be that the inhibition of prenyltransferase affects the isoprenylation of substrates, meanwhile, it may also affect the activity of HMG CoA reductase [135].
Others
Farnesyl pyrophosphates (FPPs) are fundamental enzymes in the synthesis of FPP during farnesylation [136]. As a substrate in the isoprenylation of proteins, FPP is the target of nitrogen-containing bisphosphonates [137]. In colon cancer cells, pamidronate (PAM) can inhibit the growth and induce apoptosis of tumor cells by inhibiting FPPs and blocking isoprenylation. Phosphatase of Regenerating Liver-3(PRL-3) relies on its CAAX sequence to undergo isoprenylation to enhance its ability to migrate and invade. The isoprenylation of PRL-3 alters its localization and the metastasis of gastric cancer cells, which leads to poor prognosis in patients [138]. In addition, C-isoprenylated flavonoids can promote apoptosis of colon cancer cells through increasing caspase activity [139].
Citrullination
Citrullination, which was first discovered in rheumatoid arthritis [140], refers to the process that the positively charged arginine residues are deiminated into electrically neutral citrullinine residues under the catalysis of peptidyl arginine deiminases (PADIs or PADs). This process is accompanied by the removal of amino groups, so it is also known as peptide-arginine deamination [141]. Until now, the most widely studied proteins involved in citrullination are histones. In addition, keratin 8 (KRT8), heat shock protein 60 (HSP60), α-enolase (ENO1), tubulin β (TUBB), T cell receptor β chain (TCRβ) and vimentin (VIME) are also suggested to be citrullinated proteins in tumor cells [140, 142]. Citrullination is thought to play an important role in the development of cancers because it can affect the folding state and function of proteins and interfere with protein stability.
PADIs
PADIs include PADI1-4 and PADI6, a total of five isoenzymes [143] (Table 2). In colon cancer patients, the anti-parasitic drug nitazoxanide (NTZ) targets PADI2 to stabilize PADI2, which increases the citrullination of β-catenin protein in cancer cells and blocks the Wnt/β-catenin signaling pathway, thereby inhibiting the growth of cancer cells [144]. In addition, it has been found that PADI2 can arrest the cell cycle in the G1 phase and inhibit the proliferation of colonic epithelial cells by catalyzing the citrullination, which may serve as a target for colon cancer treatment [145]. As the substrate of PADI4, the tumor suppressor protein ING4 binds to PADI4 for citrullination, which competitively destroys the affinity between ING4 and P53, affects the acetylation of P53 and inhibits the expression of P21 downstream in colorectal cancer [146]. In colorectal cancer cells, GSK3β binds to highly expressed PADI4 and is citrullinated at R344, promoting nuclear translocation of GSK3β from the cytoplasm to nucleus. Nuclear accumulation of GSK3β thus promotes ubiquitin-dependent proteasome degradation of CDKN1A, which is an important transcriptional target of p53 protein and regulatory factor of the cell cycle [147]. In addition, the components of ECM such as collagen are increased in cancers. In liver metastases of colorectal cancer, the citrullination of ECM proteins mediated by PADI4 can promote MET by enhancing the adhesion of cancer cells and reducing their migration and activity, which facilitates the growth of colorectal cancer cells that have metastasized to the liver [148].
Tumorigenesis and development
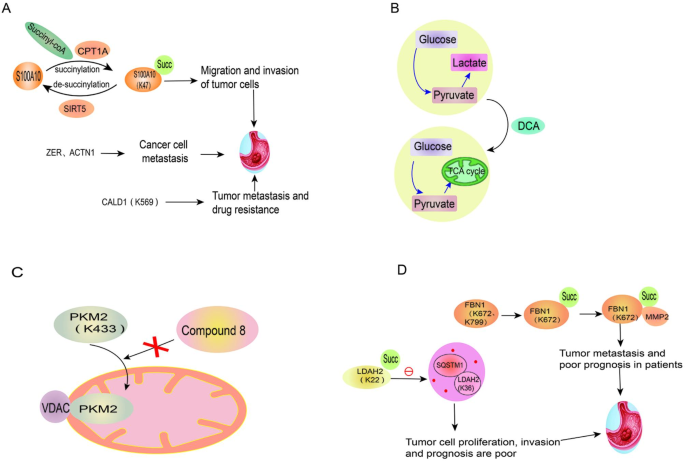
S100A10 protein, a member of the calcium-binding cytoplasmic protein family [164], was found to be succinylated at K47, which inhibits its degradation in gastric cancer [165]. Stable S100A10 could thereby promote the migration and invasion of tumor cells. By identifying the lysine group of succinylation in gastric cancer tissues, it was found that ZER, ACTN1 and other down-regulated succinylated proteins were related to the activity of cytoskeletal, and may be closely associated with the metastasis of gastric cancer. Since the actin-binding protein caldesmon (CALD1) is a succinylated protein modified at K569 and is associated with metastasis and drug resistance of gastric cancer, it might serve as a critical marker of gastric cancer. In addition, the succinylation of the proteins is also regulated by LncRNAs such as H19, CCAT1 and GAS5 [166].
Treatment
Warburg effect (also known as aerobic glycolysis) refers to the preference of malignant cells to obtain energy through glycolysis, even under aerobic conditions [167]. Among the enzymes in the TCA cycle, seven kinds of enzymes have been found to be involved in succinylation. Dichloroacetic acid (DCA), a pyruvate dehydrogenase (PDK) inhibitor can reverse the Warburg effect to normal phosphorylation, reduce the level of proteins involved in cell cycle, division and proliferation by inducing succinylation in mitochondria, and induce apoptosis [168]. Therefore, it is considered as a potential anti-tumor drug for the treatment of colorectal cancer [168].
Citrate synthase (CS), the rate-limiting enzyme of the TCA cycle, undergoes de-succinylation at the K393 and K395 sites after interacting with SIRT5. However, the highly succinylated CS at K393 and K395 inhibits colon cancer cell proliferation and migration, which provides a new target for therapy [169].
PKM2 translocated to the mitochondria is succinylated at K433 in the condition of glucose starvation, thereby binding to volt-dependent anion channel protein (VDAC) and stabilizing the VDAC protein, which increases mitochondrial permeability and contributes to tumor cell survival by inhibiting apoptosis through Bcl2. Therefore, small molecule compound 8, which could significantly reduce the activity of PKM2, was synthesized to inhibit the growth of tumors such as colon cancer by blocking the translocation of PKM2 [170].
Prognosis
High expression of fibrinogen 1 (FBN1), a major component of extracellular matrix (ECM) [171], predicts poor prognosis in patients with gastric cancer. The succinylation of FBN1 at K672 inhibits its degradation by MMP2. In addition to promoting the proliferation of gastric cancer cells by activating TGF-β1 and PI3K/Akt pathways, the accumulated FBN1 can also promote the overexpression of MMP2 by increasing the level of HIF-1α, leading to extracellular matrix protein degradation and tumor metastasis [172].
Lactate dehydrogenase A (LDHA) is a key enzyme in aerobic glycolysis mediated by CPT1A [173]. Succinylation of LDAH at K222 affects the degradation of LDHA by binding to SQSTM1 in lysosomes, and the increased LDHA contributes to the proliferation and invasion of gastric cancer cells [9]. Thus, the level of succinylated LDHA is positively associated with poor prognosis in patients with gastric cancer.
Other non-classical post-translational modifications
Palmitoylation
Palmitoylation refers to the reversible modification of saturated fatty acid palmitate to the cysteine residues of the target protein [174]. The enzyme porcupine (PORC), located in the endoplasmic reticulum, is palmitoylated on serine residues [175]. Palmitoylation of PORC regulates the secretion of Wnt, and the Wnt receptor Frizzled7 (FZD7) is essential for the reverse conversion of EMT to MET, which allows colorectal cancer to metastasize to other organs [176]. Tumornecrosisfactor-Related Apoptosis-Inducing Ligand (TRAIL), a member of the TNF protein family [177], binds to trimer death receptors 4 (DR4) and 5 (DR5) to induce tumor cell apoptosis. Colorectal cancer cells resistant to oxaliplatin are more sensitive to TRAIL, and the mechanism is that the DR4 level is up-regulated due to increased palmitoylation of DR4 and increased metastasis to lipid rafts [178]. 17 β-estradiol (E2) receptors have two types, ERα and ERβ [179]. In colon cancer cells, the palmitoylation of ERα enables its localization on the plasma membrane, which plays a crucial role in the transcription of the cyclinD1 promoter and contributes to cell proliferation. In contrast to ERα, E2 induces an increase in ERβ levels in colon cancer cells and promotes ERβ palmitoylation through the activation of the P38/MAPK pathway, and palmitoylated ERβ in turn regulates the E2-induced apoptotic process and plays an anti-proliferation role [180, 181]. In colorectal cancer, palmitoylation may also play a role in internalization initiated by the binding of monoclonal antibody (mAb) A33 to antigen (A33) [182].
Immunotherapy resistance is often associated with mutations in IFN and MHC signaling genes [183]. Optineurin, the shared node of the two signaling pathways, affects the binding of AP3D1 to IFNGR1 (IFNγ receptor), which is palmitoylated at Cys122, thereby preventing IFNGR1 from being degraded by lysosomes and maintaining the integrity of the two signaling pathways [184, 185]. Therefore, in colorectal cancer, optineurin deficiency affects the expression of both pathways and reduces cellular immunity, resulting in drug resistance. Furthermore, 2-bromopalmitate activates anti-tumor immunity in colon cancer mice by inhibiting the palmitoylation of PD-L1 or silencing DHHC3, an enzyme required for the palmitoylation of PD-L1, which provides new ideas for overcoming PD-L1-mediated immune evasion [186]. In the treatment of colon cancer, 5-FU in combination with the antimalarial drug thioridazine or the CNS drug sertraline can improve the efficacy of anti-tumor drugs. Antimalarial drugs or CNS drugs target the PPT1 and inhibit the de-palmitoylation of PPT1 enzyme to suppress the mTOR signaling pathway, thus reducing the accumulation of anti-tumor drugs in the lysosome [187].
Nitrosylation and nitration
The process of S-nitrosylation and nitration involves the addition of NO transfer to cysteine residues (S-nitrosylation) or tyrosine residues (nitration), which regulates the function of target proteins [188, 189].
NO donor anti-tumor drugs can induce cell apoptosis and inhibit cell metastasis without causing drug resistance in tumor cells [190]. Nitric oxide-donating aspirin (NO-ASA), a cancer preventive drug, promotes S-nitrosylation and tyrosine nitration of proteins in colon cancer cells by increasing NO. The mechanisms of its inhibition against colon cancer are complicated. NO donor inhibits the interaction of β-catenin with cysteine residues in the binding domain of TCF, thus inhibiting the S-nitrosylation modification of β-catenin and Wnt signaling pathway [191]. Also, NO donor inhibits the interaction of NF-kB with its homologous DNA oligonucleotides [192]. On the other hand, NO donor regulates the inactivation of NF-kB and promotes the degradation of β-catenin through tyrosine nitration in colon cancer [193]. It is worth noting that the level of nitrotyrosine is increased in colon cancer patients, and tyrosine nitration may predict the progression of cancer in patients [194]. In addition, peroxynitrite may promote the development of colorectal cancer by abnormally activating the NF-kB signaling pathway through the dual mechanisms of p38-dependent nitration and phosphorylation of IKB-α [195]. Besides, synthetic OA derivatives (SOADs) 4c can inhibit multidrug resistance (MDR) of drug-resistant cancer cells in colon cancer by inducing tyrosine residue nitration of ATP binding box (ABC) transporter protein, which is a novel treatment for drug-resistant colon cancer [196].
Summary and future directions
Post-translational modifications (PTMs) of proteins regulate multiple functions as well as contribute to the occurrence and development of tumors. Apart from canonical protein post-translational modifications such as phosphorylation, ubiquitination, methylation, glycosylation and acetylation, recent investigations of non-canonical post-translational modifications suggest their close implication in occurrence, cell proliferation, invasion, metastasis, prognosis and treatment of gastrointestinal tumors. Various non-canonical post-translational modifications involve different signaling pathways, regulatory factors and enzymes in carcinogenesis and development.
Non-canonical post-translational modifications have been reported to be closely implicated in the development of gastrointestinal tumors. Studies suggested that non-canonical PTMs such as SENP2 mediated de-SUMOylation of N-myc, Mdm2 regulated neddylation of HuR, and PADI4 promoted histone citrullination of NETs contribute to the progression of gastrointestinal tumors. Furthermore, non-canonical PTMs including GLUT1-associated lncRNAs regulated SUMOylation of GLUT1 protein, PRL-3 isoprenylation mediated translocation, and succinylated CALD1 protein lead to metastasis of gastrointestinal tumors. Moreover, it has been unraveled that different non-canonical PTMs participate in the therapy response and drug resistance of gastrointestinal tumors.
Non-canonical PTMs of proteins diversify the proteome and significantly influence the activity, localization, and functional pathways of proteins. Similar to canonical PTMs, non-canonical PTMs are of profound significance in clinical diagnosis and treatment. SUMO and NEDD8 are ubiquitin-like peptides undergoing SUMOylation and neddylation, respectively. They are conjugated by a chain reaction of E1, E2, and E3 enzymes. Chemotherapeutic agents targeting the SUMOylation and neddylation processes are being developed, of which MLN4924 is the only E1 inhibitor in Phase II clinical trials. Despite the lack of specificity and low activity, several UBC9 inhibitors have been reported, including GSK145A, 2-D08, and spectectcinB1. The expression of E3 ligase is increased in tumors and is closely related to the poor prognosis of patients. Because of its specificity, two kinds of E3 ligase-based drugs, GDC-199 and nutlins, are used in clinical trials [197, 198].
Protein lipidation refers to the process by which various lipids and their metabolites bind to and modify proteins in cells. Lipidation not only regulates protein function, but also is closely related to metabolism and cell energy homeostasis. There are four common types of lipid modification, including myristoylation, palmitoylation, isoprenylation, and glycosylphosphatidylinositol anchoring [199]. Palmitoylation is the most common lipid modification and is therefore considered important in the treatment of cancer. For example, up-regulated fatty acid synthase (which catalyzes palmitate synthesis) in cancer has been identified as a therapeutic target. At present, researches on inhibitors of protein palmitoylation has been carried out but they lack specificity [200, 201]. Ras protein is a widely studied carcinogenic protein, and isoprenylation is closely related to ras protein. In addition to statins, which are already widely used in clinical, FTIs are one of the first signal transduction inhibitors to be tested for anti-tumor clinical properties. Although the results of Phase II clinical trials are not satisfactory and could not specifically select cancer cells, it still provides an idea for clinical treatment of RAS-related tumors and some non-neoplastic diseases, such as diabetic retinopathy and macular disease [202]. Isoprenyl carboxy methyltransferase inhibitors have been reported as chemotherapeutic agents targeting the ras signaling pathway, including cysmethynil, AdoHcy, AFC, and AGGC, but these inhibitors need to be further optimized before clinical use [203].
Citrullination of histones can be used as biomarkers and clinical therapeutic targets. For advanced cancer patients, CitH3 can be used to assess their inflammatory response and predict the occurrence of venous thromboembolism. By targeting citH3 and NET, compounds commonly used in herbal medicine can inhibit hematogenous metastasis of certain tumors. Given that citrullination is mediated by PAD, PAD inhibitors include reversible inhibitors (taxol, minocycline, streptomycin, SC97362, Inh-Dap) and irreversible inhibitors (NSC95397, streptonigrin, cl-amidine, F-amidine, O-cl) amidine, O-F-amidine, TDFA ) are investigated in order to overcome the defects of poor selectivity and low bioavailability [204, 205].
Studies on sumoylation, succinylation, neddylation, Isoprenylation, citrullination, nitrification, and nitrosation suggested promising application of non-canonical PTMs in clinical diagnosis, treatment and drug resistance of patients with gastrointestinal tumors. Other novel non-canonical PTMs such as ribosylation, formylation, O-GlcNAcylation, deamination, propionylation and butyrylation are currently poorly understood. In addition, specific mechanisms and clinical applications of non-classical PTMs have not been thoroughly studied. Further studies are required to elucidate the therapeutic potential of targeting non-classical PTMs to improve the treatment and prognosis of gastrointestinal tumors.
Data availability
All the data used in the manuscript are freely available online.
References
- Prabakaran S, Lippens G, Steen H, Gunawardena J. Post-translational modification: nature’s escape from genetic imprisonment and the basis for dynamic information encoding. Wiley Interdisciplinary Reviews Systems Biology and Medicine. 2012;4(6):565–83. PubMedPubMed CentralCASGoogle Scholar
- Czuba LC, Hillgren KM, Swaan PW. Post-translational modifications of transporters. Pharmacol Ther. 2018;192:88–99. PubMedPubMed CentralCASGoogle Scholar
- Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr. Protein posttranslational modifications: the chemistry of proteome diversifications. Angewandte Chemie (International ed in English). 2005;44(45):7342–72. PubMedCASGoogle Scholar
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. Google Scholar
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: an overview. Int J Cancer 2021.
- Peng Y, Zhang Z, Zhang A, Liu C, Sun Y, Peng Z, Liu Y. Membrane-cytoplasm translocation of annexin A4 is involved in the metastasis of colorectal carcinoma. Aging. 2021;13(7):10312–25. PubMedPubMed CentralCASGoogle Scholar
- Wu Y, Tedesco L, Lucia K, Schlitter AM, Garcia JM, Esposito I, Auernhammer CJ, Theodoropoulou M, Arzt E, Renner U, et al. RSUME is implicated in tumorigenesis and metastasis of pancreatic neuroendocrine tumors. Oncotarget. 2016;7(36):57878–93. PubMedPubMed CentralGoogle Scholar
- Lan H, Tang Z, Jin H, Sun Y. Neddylation inhibitor MLN4924 suppresses growth and migration of human gastric cancer cells. Sci Rep. 2016;6:24218. PubMedPubMed CentralCASGoogle Scholar
- Li X, Zhang C, Zhao T, Su Z, Li M, Hu J, Wen J, Shen J, Wang C, Pan J, et al. Lysine-222 succinylation reduces lysosomal degradation of lactate dehydrogenase a and is increased in gastric cancer. J Experimental Clin cancer Research: CR. 2020;39(1):172. PubMed CentralCASGoogle Scholar
- Eifler K, Vertegaal ACO. SUMOylation-Mediated regulation of cell cycle progression and Cancer. Trends Biochem Sci. 2015;40(12):779–93. PubMedPubMed CentralCASGoogle Scholar
- Han ZJ, Feng YH, Gu BH, Li YM, Chen H. The post-translational modification, SUMOylation, and cancer (review). Int J Oncol. 2018;52(4):1081–94. PubMedPubMed CentralCASGoogle Scholar
- Kunz K, Piller T, Müller S. SUMO-specific proteases and isopeptidases of the SENP family at a glance. J Cell Sci 2018, 131(6).
- Guo C, Henley JM. Wrestling with stress: roles of protein SUMOylation and deSUMOylation in cell stress response. IUBMB Life. 2014;66(2):71–7. PubMedCASGoogle Scholar
- Xu Y, Li J, Zuo Y, Deng J, Wang LS, Chen GQ. SUMO-specific protease 1 regulates the in vitro and in vivo growth of colon cancer cells with the upregulated expression of CDK inhibitors. Cancer Lett. 2011;309(1):78–84. PubMedCASGoogle Scholar
- Xianjun F, Xirui X, Jie T, Huiwen M, Shaojun Z, Qiaoyun L, Yunxin L, Xuqun S. Momordin Ic induces G0/1 phase arrest and apoptosis in colon cancer cells by suppressing SENP1/c-MYC signaling pathway. J Pharmacol Sci. 2021;146(4):249–58. PubMedGoogle Scholar
- Hu XY, Liu Z, Zhang KL, Feng J, Liu XF, Wang LY, Wang ZW. SUMO-specific protease 2-mediated deSUMOylation is required for NDRG2 stabilization in gastric cancer cells. Cancer Biomark A. 2017;21(1):195–201. Google Scholar
- Ren YH, Liu KJ, Wang M, Yu YN, Yang K, Chen Q, Yu B, Wang W, Li QW, Wang J et al. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells Oncotarget 2014, 5(16):7093–104.
- Song JG, Xie HH, Li N, Wu K, Qiu JG, Shen DM, Huang CJ. SUMO-specific protease 6 promotes gastric cancer cell growth via deSUMOylation of FoxM1. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(12):9865–71. PubMedCASGoogle Scholar
- Wang PS, Wang Z, Yang C. Dysregulations of long non-coding RNAs - the emerging lnc in environmental carcinogenesis. Sem Cancer Biol. 2021;76:163–72. CASGoogle Scholar
- Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Reviews Clin Oncol. 2022;19(3):188–206. CASGoogle Scholar
- Han J, Nie M, Chen C, Cheng X, Guo T, Huangfu L, Li X, Du H, Xing X, Ji J. SDCBP-AS1 destabilizes β-catenin by regulating ubiquitination and SUMOylation of hnRNP K to suppress gastric tumorigenicity and metastasis. Cancer Commun (London England). 2022;42(11):1141–61. Google Scholar
- Huang G, Cai G, Hu D, Li J, Xu Q, Chen Z, Xu B. Low SP1 SUMOylation-dependent SNHG17 upregulation promotes drug resistance of gastric cancer through impairing hsa-miR-23b-3p-induced Notch2 inhibition. Cell Oncol (Dordrecht) 2022.
- Mei D, Song H, Wang K, Lou Y, Sun W, Liu Z, Ding X, Guo J. Up-regulation of SUMO1 pseudogene 3 (SUMO1P3) in gastric cancer and its clinical association. Med Oncol (Northwood Lond Engl). 2013;30(4):709. Google Scholar
- Li B, Kang H, Xiao Y, Du Y, Xiao Y, Song G, Zhang Y, Guo Y, Yang F, He F, et al. LncRNA GAL promotes colorectal cancer liver metastasis through stabilizing GLUT1. Oncogene. 2022;41(13):1882–94. PubMedCASGoogle Scholar
- Chen X, Liu K, Xu W, Zhou G, Yuan C. Tumor-related Molecular Regulatory Mechanisms of Long non-coding RNA RMST: recent evidence. Mini Rev Med Chem. 2022;22(10):1374–9. PubMedCASGoogle Scholar
- Peng C, Tan Y, Yang P, Jin K, Zhang C, Peng W, Wang L, Zhou J, Chen R, Wang T, et al. Circ-GALNT16 restrains colorectal cancer progression by enhancing the SUMOylation of hnRNPK. J Experimental Clin cancer Research: CR. 2021;40(1):272. PubMed CentralCASGoogle Scholar
- Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharmaceutics: Official J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2015;93:52–79. Google Scholar
- Liu Q, Huang Q, Liu H, He FJ, Liu JH, Zhou YY, Zeng MT, Pei Q, Zhu H. SUMOylation of methyltransferase-like 3 facilitates colorectal cancer progression by promoting circ_0000677 in an m(6) A-dependent manner. J Gastroenterol Hepatol. 2022;37(4):700–13. PubMedCASGoogle Scholar
- Oliveres H, Pesántez D, Maurel J. Lessons to learn for adequate targeted Therapy Development in Metastatic Colorectal Cancer Patients. Int J Mol Sci 2021, 22(9).
- Wagner T, Kiweler N, Wolff K, Knauer SK, Brandl A, Hemmerich P, Dannenberg JH, Heinzel T, Schneider G, Krämer OH. Sumoylation of HDAC2 promotes NF-κB-dependent gene expression. Oncotarget. 2015;6(9):7123–35. PubMedPubMed CentralGoogle Scholar
- Enzinger PC, Ilson DH, Saltz LB, O’Reilly EM, Kelsen DP. Irinotecan and cisplatin in upper gastrointestinal malignancies. Oncol (Williston Park NY). 1998;12(8 Suppl 6):110–3. CASGoogle Scholar
- Chen MC, Nhan DC, Hsu CH, Wang TF, Li CC, Ho TJ, Mahalakshmi B, Chen MC, Yang LY, Huang CY. SENP1 participates in Irinotecan resistance in human colon cancer cells. J Cell Biochem. 2021;122(10):1277–94. PubMedCASGoogle Scholar
- Prasad S, Gupta SC, Pandey MK, Tyagi AK, Deb L. Oxidative stress and Cancer: advances and Challenges. Oxidative Med Cell Longev. 2016;2016:5010423. Google Scholar
- De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol (London England). 2005;1(6):779–86. Google Scholar
- Zhou B, Zhu Y, Xu W, Zhou Q, Tan L, Zhu L, Chen H, Feng L, Hou T, Wang X, et al. Hypoxia stimulates SUMOylation-Dependent stabilization of KDM5B. Front cell Dev Biology. 2021;9:741736. Google Scholar
- Wang Q, Xu C, Fan Q, Yuan H, Zhang X, Chen B, Cai R, Zhang Y, Lin M, Xu M. Positive feedback between ROS and cis-axis of PIASxα/p38α-SUMOylation/MK2 facilitates gastric cancer metastasis. Cell Death Dis. 2021;12(11):986. PubMedPubMed CentralCASGoogle Scholar
- Hu Z, Teng XL, Zhang T, Yu X, Ding R, Yi J, Deng L, Wang Z, Zou Q. SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function. Mol Cell. 2021;81(5):940–952e945. PubMedCASGoogle Scholar
- Kang X, Li J, Zou Y, Yi J, Zhang H, Cao M, Yeh ET, Cheng J. PIASy stimulates HIF1α SUMOylation and negatively regulates HIF1α activity in response to hypoxia. Oncogene. 2010;29(41):5568–78. PubMedCASGoogle Scholar
- Lu Z, Wu H, Mo YY. Regulation of bcl-2 expression by Ubc9. Exp Cell Res. 2006;312(10):1865–75. PubMedCASGoogle Scholar
- Lu SC, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012;92(4):1515–42. PubMedCASGoogle Scholar
- Tomasi ML, Ryoo M, Skay A, Tomasi I, Giordano P, Mato JM, Lu SC. Polyamine and methionine adenosyltransferase 2A crosstalk in human colon and liver cancer. Exp Cell Res. 2013;319(12):1902–11. PubMedPubMed CentralCASGoogle Scholar
- Tomasi ML, Ryoo M, Ramani K, Tomasi I, Giordano P, Mato JM, Lu SC. Methionine adenosyltransferase α2 sumoylation positively regulate Bcl-2 expression in human colon and liver cancer cells. Oncotarget. 2015;6(35):37706–23. PubMedPubMed CentralGoogle Scholar
- López I, Chalatsi E, Ellenbroek SIJ, Andrieux A, Roux PF, Cerapio JP, Jouvion G, van Rheenen J, Seeler JS, Dejean A. An unanticipated tumor-suppressive role of the SUMO pathway in the intestine unveiled by Ubc9 haploinsufficiency. Oncogene. 2020;39(43):6692–703. PubMedPubMed CentralGoogle Scholar
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9. PubMedPubMed CentralCASGoogle Scholar
- Yu B, Swatkoski S, Holly A, Lee LC, Giroux V, Lee CS, Hsu D, Smith JL, Yuen G, Yue J, et al. Oncogenesis driven by the Ras/Raf pathway requires the SUMO E2 ligase Ubc9. Proc Natl Acad Sci USA. 2015;112(14):E1724–1733. PubMedPubMed CentralCASGoogle Scholar
- Morales M, Xue X. Targeting iron metabolism in cancer therapy. Theranostics. 2021;11(17):8412–29. PubMedPubMed CentralCASGoogle Scholar
- Birladeanu AM, Rogalska M, Potiri M, Papadaki V, Andreadou M, Kontoyiannis DL, Lewis JD, Erpapazoglou Z, Kafasla P. The scaffold protein IQGAP1 links heat-induced stress signals to alternative splicing regulation in gastric cancer cells. Oncogene. 2021;40(36):5518–32. PubMedCASGoogle Scholar
- Liang Z, Yang Y, He Y, Yang P, Wang X, He G, Zhang P, Zhu H, Xu N, Zhao X, et al. SUMOylation of IQGAP1 promotes the development of colorectal cancer. Cancer Lett. 2017;411:90–9. PubMedCASGoogle Scholar
- Tsuchiya M, Misaka R, Nitta K, Tsuchiya K. Transcriptional factors, Mafs and their biological roles. World J Diabetes. 2015;6(1):175–83. PubMedPubMed CentralGoogle Scholar
- Yang LS, Zhang XJ, Xie YY, Sun XJ, Zhao R, Huang QH. SUMOylated MAFB promotes colorectal cancer tumorigenesis. Oncotarget. 2016;7(50):83488–501. PubMedPubMed CentralGoogle Scholar
- Song N, Gu x, Wang Y, Chen Z, Shi L. [Lentivirus-mediated siRNA targeting sae1 induces cell cycle arrest and apop- tosis in colon cancer cell RKO]. Mol Biol. 2014;48(1):107–16. CASGoogle Scholar
- Truong K, Lee TD, Li B, Chen Y. Sumoylation of SAE2 C terminus regulates SAE nuclear localization. J Biol Chem. 2012;287(51):42611–9. PubMedPubMed CentralCASGoogle Scholar
- He P, Sun X, Cheng HJ, Zou YB, Wang Q, Zhou CL, Liu WQ, Hao YM, Meng XW. UBA2 promotes proliferation of colorectal cancer. Mol Med Rep. 2018;18(6):5552–62. PubMedPubMed CentralCASGoogle Scholar
- Du L, Fakih MG, Rosen ST, Chen Y. SUMOylation of E2F1 regulates expression of EZH2. Cancer Res. 2020;80(19):4212–23. PubMedPubMed CentralCASGoogle Scholar
- Lim JR, Mouawad J, Gorton OK, Bubb WA, Kwan AH. Cancer stem cell characteristics and their potential as therapeutic targets. Med Oncol (Northwood Lond Engl). 2021;38(7):76. Google Scholar
- Bogachek MV, De Andrade JP, Weigel RJ. Regulation of epithelial-mesenchymal transition through SUMOylation of transcription factors. Cancer Res. 2015;75(1):11–5. PubMedCASGoogle Scholar
- Eloranta JJ, Hurst HC. Transcription factor AP-2 interacts with the SUMO-conjugating enzyme UBC9 and is sumolated in vivo. J Biol Chem. 2002;277(34):30798–804. PubMedCASGoogle Scholar
- Bogachek MV, Park JM, De Andrade JP, Lorenzen AW, Kulak MV, White JR, Gu VW, Wu VT, Weigel RJ. Inhibiting the SUMO pathway represses the Cancer Stem Cell Population in breast and colorectal carcinomas. Stem cell Reports. 2016;7(6):1140–51. PubMedPubMed CentralCASGoogle Scholar
- Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, McDonnell DP. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103(31):11707–12. PubMedPubMed CentralCASGoogle Scholar
- Du L, Li YJ, Fakih M, Wiatrek RL, Duldulao M, Chen Z, Chu P, Garcia-Aguilar J, Chen Y. Role of SUMO activating enzyme in cancer stem cell maintenance and self-renewal. Nat Commun. 2016;7:12326. PubMedPubMed CentralCASGoogle Scholar
- Shitashige M, Satow R, Honda K, Ono M, Hirohashi S, Yamada T. Regulation of wnt signaling by the nuclear pore complex. Gastroenterology. 2008;134(7):1961–71. 1971.e1961-1964. PubMedCASGoogle Scholar
- Li L, Duan Q, Zeng Z, Zhao J, Lu J, Sun J, Zhang J, Siwko S, Wong J, Shi T, et al. UHRF2 promotes intestinal tumorigenesis through stabilization of TCF4 mediated Wnt/β-catenin signaling. Int J Cancer. 2020;147(8):2239–52. PubMedCASGoogle Scholar
- Chen C, Sun X, Xie W, Chen S, Hu Y, Xing D, Xu J, Chen X, Zhao Z, Han Z, et al. Opposing biological functions of the cytoplasm and nucleus DAXX modified by SUMO-2/3 in gastric cancer. Cell Death Dis. 2020;11(7):514. PubMedPubMed CentralCASGoogle Scholar
- Dai W, Xie S, Chen C, Choi BH. Ras sumoylation in cell signaling and transformation. Sem Cancer Biol. 2021;76:301–9. CASGoogle Scholar
- Zhang H, Kuai X, Ji Z, Li Z, Shi R. Over-expression of small ubiquitin-related modifier-1 and sumoylated p53 in colon cancer. Cell Biochem Biophys. 2013;67(3):1081–7. PubMedCASGoogle Scholar
- Du JX, Bialkowska AB, McConnell BB, Yang VW. SUMOylation regulates nuclear localization of Krüppel-like factor 5. J Biol Chem. 2008;283(46):31991–2002. PubMedPubMed CentralCASGoogle Scholar
- Luo Y, You S, Wang J, Fan S, Shi J, Peng A, Yu T. Association between Sumoylation-Related gene rs77447679 polymorphism and risk of gastric Cancer (GC) in a Chinese Population. J Cancer. 2017;8(16):3226–31. PubMedPubMed CentralGoogle Scholar
- Liu C, Wang X, Qin W, Tu J, Li C, Zhao W, Ma L, Liu B, Qiu H, Yuan X. Combining radiation and the ATR inhibitor berzosertib activates STING signaling and enhances immunotherapy via inhibiting SHP1 function in colorectal cancer. Cancer Commun (London England). 2023;43(4):435–54. Google Scholar
- Ma X, Jia S, Wang G, Liang M, Guo T, Du H, Li S, Li X, Huangfu L, Guo J, et al. TRIM28 promotes the escape of gastric cancer cells from immune surveillance by increasing PD-L1 abundance. Signal Transduct Target Therapy. 2023;8(1):246. CASGoogle Scholar
- Wu Q, Lin XF, Ye XF, Zhang B, Xie Z, Su WJ. Ubiquitinated or sumoylated retinoic acid receptor alpha determines its characteristic and interacting model with retinoid X receptor alpha in gastric and breast cancer cells. J Mol Endocrinol. 2004;32(3):595–613. PubMedCASGoogle Scholar
- Reid R, Greene PJ, Santi DV. Exposition of a family of RNA m(5)C methyltransferases from searching genomic and proteomic sequences. Nucleic Acids Res. 1999;27(15):3138–45. PubMedPubMed CentralCASGoogle Scholar
- Hu Y, Chen C, Tong X, Chen S, Hu X, Pan B, Sun X, Chen Z, Shi X, Hu Y, et al. NSUN2 modified by SUMO-2/3 promotes gastric cancer progression and regulates mRNA m5C methylation. Cell Death Dis. 2021;12(9):842. PubMedPubMed CentralCASGoogle Scholar
- Qi QM, Xue YC, Lv J, Sun D, Du JX, Cai SQ, Li YH, Gu TC, Wang MB. Ginkgolic acids induce HepG2 cell death via a combination of apoptosis, autophagy and the mitochondrial pathway. Oncol Lett. 2018;15(5):6400–8. PubMedPubMed CentralGoogle Scholar
- Liu D, Li Z, Yang Z, Ma J, Mai S. Ginkgoic acid impedes gastric cancer cell proliferation, migration and EMT through inhibiting the SUMOylation of IGF-1R. Chemico-Biol Interact. 2021;337:109394. CASGoogle Scholar
- Zhou L, Jiang Y, Luo Q, Li L, Jia L. Neddylation: a novel modulator of the tumor microenvironment. Mol Cancer. 2019;18(1):77. PubMedPubMed CentralGoogle Scholar
- Wang SY, Liu X, Liu Y, Zhang HY, Zhang YB, Liu C, Song J, Niu JB, Zhang SY. Review of NEDDylation inhibition activity detection methods. Bioorg Med Chem. 2021;29:115875. PubMedCASGoogle Scholar
- Zheng N, Shabek N. Ubiquitin ligases: structure, function, and Regulation. Annu Rev Biochem. 2017;86:129–57. PubMedCASGoogle Scholar
- Embade N, Fernández-Ramos D, Varela-Rey M, Beraza N, Sini M, Gutiérrez de Juan V, Woodhoo A, Martínez-López N, Rodríguez-Iruretagoyena B, Bustamante FJ, et al. Murine double minute 2 regulates Hu antigen R stability in human liver and colon cancer through NEDDylation. Hepatology (Baltimore MD). 2012;55(4):1237–48. PubMedCASGoogle Scholar
- Xu S, Ma Y, Tong Q, Yang J, Liu J, Wang Y, Li G, Zeng J, Fang S, Li F, et al. Cullin-5 neddylation-mediated NOXA degradation is enhanced by PRDX1 oligomers in colorectal cancer. Cell Death Dis. 2021;12(3):265. PubMedPubMed CentralCASGoogle Scholar
- Jumpertz S, Hennes T, Asare Y, Schütz AK, Bernhagen J. CSN5/JAB1 suppresses the WNT inhibitor DKK1 in colorectal cancer cells. Cell Signal. 2017;34:38–46. PubMedCASGoogle Scholar
- Jang SM, Redon CE, Thakur BL, Bahta MK, Aladjem MI. Regulation of cell cycle drivers by Cullin-RING ubiquitin ligases. Exp Mol Med. 2020;52(10):1637–51. PubMedPubMed CentralCASGoogle Scholar
- Jia L, Sun Y. SCF E3 ubiquitin ligases as anticancer targets. Curr Cancer Drug Targets. 2011;11(3):347–56. PubMedPubMed CentralCASGoogle Scholar
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34(4):176–88. PubMedCASGoogle Scholar
- Gong Y, Xiang XJ, Feng M, Chen J, Fang ZL, Xiong JP. CUL4A promotes cell invasion in gastric cancer by activating the NF-κB signaling pathway. Biologics: Targets & Therapy. 2017;11:45–53. CASGoogle Scholar
- Shamay M, Greenway M, Liao G, Ambinder RF, Hayward SD. De novo DNA methyltransferase DNMT3b interacts with NEDD8-modified proteins. J Biol Chem. 2010;285(47):36377–86. PubMedPubMed CentralCASGoogle Scholar
- Zhu Y, Li L, Hou D, Ouyang Y, Guo X, Wang Y, Li J, Gong K. MicroRNA-19a regulates the proliferation, migration and invasion of human gastric cancer cells by targeting CUL5. Arch Biochem Biophys. 2019;662:93–100. PubMedCASGoogle Scholar
- Enchev RI, Schulman BA, Peter M. Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol. 2015;16(1):30–44. PubMedPubMed CentralCASGoogle Scholar
- Liang Q, Liu M, Li J, Tong R, Hu Y, Bai L, Shi J. NAE modulators: a potential therapy for gastric carcinoma. Eur J Med Chem. 2022;231:114156. PubMedCASGoogle Scholar
- Lockhart AC, Bauer TM, Aggarwal C, Lee CB, Harvey RD, Cohen RB, Sedarati F, Nip TK, Faessel H, Dash AB, et al. Phase ib study of pevonedistat, a NEDD8-activating enzyme inhibitor, in combination with docetaxel, carboplatin and paclitaxel, or gemcitabine, in patients with advanced solid tumors. Investig New Drugs. 2019;37(1):87–97. CASGoogle Scholar
- Zhang Q, Hou D, Luo Z, Chen P, Lv B, Wu L, Ma Y, Chu Y, Liu H, Liu F, et al. The novel protective role of P27 in MLN4924-treated gastric cancer cells. Cell Death Dis. 2015;6(8):e1867. PubMedPubMed CentralCASGoogle Scholar
- Hu L, Bai ZG, Ma XM, Bai N, Zhang ZT. MRFAP1 plays a protective role in neddylation inhibitor MLN4924-mediated gastric cancer cell death. Eur Rev Med Pharmacol Sci. 2018;22(23):8273–80. PubMedCASGoogle Scholar
- Lin A, Zhang J, Luo P. Crosstalk between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front Immunol. 2020;11:2039. PubMedPubMed CentralCASGoogle Scholar
- McGrail DJ, Garnett J, Yin J, Dai H, Shih DJH, Lam TNA, Li Y, Sun C, Li Y, Schmandt R, et al. Proteome instability is a therapeutic vulnerability in Mismatch Repair-Deficient Cancer. Cancer Cell. 2020;37(3):371–386e312. PubMedPubMed CentralCASGoogle Scholar
- Kanapathipillai M. Treating p53 mutant Aggregation-Associated Cancer. Cancers 2018, 10(6).
- Wu KJ, Zhong HJ, Li G, Liu C, Wang HD, Ma DL, Leung CH. Structure-based identification of a NEDD8-activating enzyme inhibitor via drug repurposing. Eur J Med Chem. 2018;143:1021–7. PubMedCASGoogle Scholar
- Song J, Cui XX, Wu BW, Li D, Wang SH, Shi L, Zhu T, Zhang YB, Zhang SY. Discovery of 1,2,4-triazine-based derivatives as novel neddylation inhibitors and anticancer activity studies against gastric cancer MGC-803 cells. Bioorg Med Chem Lett. 2020;30(2):126791. PubMedCASGoogle Scholar
- Fu DJ, Cui XX, Zhu T, Zhang YB, Hu YY, Zhang LR, Wang SH, Zhang SY. Discovery of novel indole derivatives that inhibit NEDDylation and MAPK pathways against gastric cancer MGC803 cells. Bioorg Chem. 2021;107:104634. PubMedCASGoogle Scholar
- Wang B, Zhang QH, Li XJ, Wang SQ, Chen XB, Yu B, Liu HM. Discovery of a cinnamyl piperidine derivative as new neddylation inhibitor for gastric cancer treatment. Eur J Med Chem. 2021;226:113896. PubMedCASGoogle Scholar
- Fu DJ, Song J, Zhu T, Pang XJ, Wang SH, Zhang YB, Wu BW, Wang JW, Zi X, Zhang SY, et al. Discovery of novel tertiary amide derivatives as NEDDylation pathway activators to inhibit the tumor progression in vitro and in vivo. Eur J Med Chem. 2020;192:112153. PubMedCASGoogle Scholar
- Song J, Liu Y, Yuan XY, Liu WB, Li YR, Yu GX, Tian XY, Zhang YB, Fu XJ, Zhang SY. Discovery of 1,2,4-triazine dithiocarbamate derivatives as NEDDylation agonists to inhibit gastric cancers. Eur J Med Chem. 2021;225:113801. PubMedCASGoogle Scholar
- Li HL, Li QY, Jin MJ, Lu CF, Mu ZY, Xu WY, Song J, Zhang Y, Zhang SY. A review: hippo signaling pathway promotes tumor invasion and metastasis by regulating target gene expression. J Cancer Res Clin Oncol. 2021;147(6):1569–85. PubMedGoogle Scholar
- Xiao J, Li G, Zhou J, Wang S, Liu D, Shu G, Zhou J, Ren F. MicroRNA-520b functions as a tumor suppressor in Colorectal Cancer by inhibiting defective in Cullin Neddylation 1 Domain containing 1 (DCUN1D1). Oncol Res. 2018;26(4):593–604. PubMedPubMed CentralGoogle Scholar
- Noguchi K, Okumura F, Takahashi N, Kataoka A, Kamiyama T, Todo S, Hatakeyama S. TRIM40 promotes neddylation of IKKγ and is downregulated in gastrointestinal cancers. Carcinogenesis. 2011;32(7):995–1004. PubMedCASGoogle Scholar
- Xie P, Zhang M, He S, Lu K, Chen Y, Xing G, Lu Y, Liu P, Li Y, Wang S, et al. The covalent modifier Nedd8 is critical for the activation of Smurf1 ubiquitin ligase in tumorigenesis. Nat Commun. 2014;5:3733. PubMedCASGoogle Scholar
- Du MG, Liu F, Chang Y, Tong S, Liu W, Chen YJ, Xie P. Neddylation modification of the U3 snoRNA-binding protein RRP9 by Smurf1 promotes tumorigenesis. J Biol Chem. 2021;297(5):101307. PubMedPubMed CentralCASGoogle Scholar
- Jeong A, Suazo KF, Wood WG, Distefano MD, Li L. Isoprenoids and protein prenylation: implications in the pathogenesis and therapeutic intervention of Alzheimer’s disease. Crit Rev Biochem Mol Biol. 2018;53(3):279–310. PubMedPubMed CentralGoogle Scholar
- Longo DL, Rosen N. Targeting oncogenic RAS protein. N Engl J Med. 2022;387(2):184–6. PubMedGoogle Scholar
- Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell. 2005;7(4):297–300. PubMedCASGoogle Scholar
- Bagchi S, Rathee P, Jayaprakash V, Banerjee S. Farnesyl transferase inhibitors as potential Anticancer Agents. Mini Rev Med Chem. 2018;18(19):1611–23. PubMedCASGoogle Scholar
- Cengel KA, Voong KR, Chandrasekaran S, Maggiorella L, Brunner TB, Stanbridge E, Kao GD, McKenna WG, Bernhard EJ. Oncogenic K-Ras signals through epidermal growth factor receptor and wild-type H-Ras to promote radiation survival in pancreatic and colorectal carcinoma cells. Neoplasia (New York NY). 2007;9(4):341–8. CASGoogle Scholar
- Liu M, Bryant MS, Chen J, Lee S, Yaremko B, Li Z, Dell J, Lipari P, Malkowski M, Prioli N, et al. Effects of SCH 59228, an orally bioavailable farnesyl protein transferase inhibitor, on the growth of oncogene-transformed fibroblasts and a human colon carcinoma xenograft in nude mice. Cancer Chemother Pharmacol. 1999;43(1):50–8. PubMedCASGoogle Scholar
- Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997;272(22):14459–64. PubMedCASGoogle Scholar
- Krens LL, Baas JM, Gelderblom H, Guchelaar HJ. Therapeutic modulation of k-ras signaling in colorectal cancer. Drug Discovery Today. 2010;15(13–14):502–16. PubMedCASGoogle Scholar
- Di Paolo A, Danesi R, Nardini D, Bocci G, Innocenti F, Fogli S, Barachini S, Marchetti A, Bevilacqua G, Del Tacca M. Manumycin inhibits ras signal transduction pathway and induces apoptosis in COLO320-DM human colon tumour cells. Br J Cancer. 2000;82(4):905–12. PubMedPubMed CentralGoogle Scholar
- Nagase T, Kawata S, Tamura S, Matsuda Y, Inui Y, Yamasaki E, Ishiguro H, Ito T, Miyagawa J, Mitsui H, et al. Manumycin and gliotoxin derivative KT7595 block ras farnesylation and cell growth but do not disturb lamin farnesylation and localization in human tumour cells. Br J Cancer. 1997;76(8):1001–10. PubMedPubMed CentralCASGoogle Scholar
- Murphy LA, Moore T, Nesnow S. Propiconazole-enhanced hepatic cell proliferation is associated with dysregulation of the cholesterol biosynthesis pathway leading to activation of Erk1/2 through ras farnesylation. Toxicol Appl Pharmcol. 2012;260(2):146–54. CASGoogle Scholar
- Di Paolo A, Danesi R, Caputo S, Macchia M, Lastella M, Boggi U, Mosca F, Marchetti A, Del Tacca M. Inhibition of protein farnesylation enhances the chemotherapeutic efficacy of the novel geranylgeranyltransferase inhibitor BAL9611 in human colon cancer cells. Br J Cancer. 2001;84(11):1535–43. PubMedPubMed CentralGoogle Scholar
- Broniarek I, Jarmuszkiewicz W. [Statins and mitochondria]. Postepy Biochem. 2016;62(2):77–84. PubMedGoogle Scholar
- Sillero MA, de Diego A, Tavares JE, Silva JA, Pérez-Zúñiga FJ, Sillero A. Synthesis of ATP derivatives of compounds of the mevalonate pathway (isopentenyl di- and triphosphate; geranyl di- and triphosphate, farnesyl di- and triphosphate, and dimethylallyl diphosphate) catalyzed by T4 RNA ligase, T4 DNA ligase and other ligases potential relationship with the effect of bisphosphonates on osteoclasts. Biochem Pharmacol. 2009;78(4):335–43. PubMedCASGoogle Scholar
- Ravnskov U, McCully KS, Rosch PJ. The statin-low cholesterol-cancer conundrum. QJM: Monthly Journal of the Association of Physicians. 2012;105(4):383–8. PubMedCASGoogle Scholar
- Vallianou NG, Kostantinou A, Kougias M, Kazazis C. Statins and cancer. Anti-cancer Agents Med Chem. 2014;14(5):706–12. CASGoogle Scholar
- Theodosakis N, Langdon CG, Micevic G, Krykbaeva I, Means RE, Stern DF, Bosenberg MW. Inhibition of isoprenylation synergizes with MAPK blockade to prevent growth in treatment-resistant melanoma, colorectal, and lung cancer. Pigment cell & Melanoma Research. 2019;32(2):292–302. CASGoogle Scholar
- Chidharla A, Parsi M, Kasi A. Cetuximab. StatPearls Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.
- Krens LL, Simkens LH, Baas JM, Koomen ER, Gelderblom H, Punt CJ, Guchelaar HJ. Statin use is not associated with improved progression free survival in cetuximab treated KRAS mutant metastatic colorectal cancer patients: results from the CAIRO2 study. PLoS ONE. 2014;9(11):e112201. PubMedPubMed CentralGoogle Scholar
- Baas JM, Krens LL, ten Tije AJ, Erdkamp F, van Wezel T, Morreau H, Gelderblom H, Guchelaar HJ. Safety and efficacy of the addition of simvastatin to cetuximab in previously treated KRAS mutant metastatic colorectal cancer patients. Investig New Drugs. 2015;33(6):1242–7. CASGoogle Scholar
- Narisawa T, Morotomi M, Fukaura Y, Hasebe M, Ito M, Aizawa R. Chemoprevention by pravastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, of N-methyl-N-nitrosourea-induced colon carcinogenesis in F344 rats. Japanese J cancer Research: Gann. 1996;87(8):798–804. CASGoogle Scholar
- Iishi H, Tatsuta M, Baba M, Yano H, Sakai N, Uehara H, Nakaizumi A. Ras p21 isoprenylation inhibition induces flat colon tumors in Wistar rats. Dis Colon Rectum. 2000;43(1):70–5. PubMedCASGoogle Scholar
- Menter DG, Ramsauer VP, Harirforoosh S, Chakraborty K, Yang P, Hsi L, Newman RA, Krishnan K. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PLoS ONE. 2011;6(12):e28813. PubMedPubMed CentralCASGoogle Scholar
- Kaneko R, Tsuji N, Asanuma K, Tanabe H, Kobayashi D, Watanabe N. Survivin down-regulation plays a crucial role in 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor-induced apoptosis in cancer. J Biol Chem. 2007;282(27):19273–81. PubMedCASGoogle Scholar
- Agarwal B, Bhendwal S, Halmos B, Moss SF, Ramey WG, Holt PR. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin cancer Research: Official J Am Association Cancer Res. 1999;5(8):2223–9. CASGoogle Scholar
- Klein CH, Truxius DC, Vogel HA, Harizanova J, Murarka S, Martín-Gago P, Bastiaens PIH. PDEδ inhibition impedes the proliferation and survival of human colorectal cancer cell lines harboring oncogenic KRas. Int J Cancer. 2019;144(4):767–76. PubMedCASGoogle Scholar
- Gillette WK, Esposito D, Abreu Blanco M, Alexander P, Bindu L, Bittner C, Chertov O, Frank PH, Grose C, Jones JE, et al. Farnesylated and methylated KRAS4b: high yield production of protein suitable for biophysical studies of prenylated protein-lipid interactions. Sci Rep. 2015;5:15916. PubMedPubMed CentralCASGoogle Scholar
- Amissah F, Duverna R, Aguilar BJ, Poku RA, Lamango NS. Polyisoprenylated methylated protein methyl esterase is both sensitive to curcumin and overexpressed in colorectal cancer: implications for chemoprevention and treatment. Biomed Res Int. 2013;2013:416534. PubMedPubMed CentralGoogle Scholar
- Schulz S, Nyce JW. Inhibition of protein isoprenylation and p21ras membrane association by dehydroepiandrosterone in human colonic adenocarcinoma cells in vitro. Cancer Res. 1991;51(24):6563–7. PubMedCASGoogle Scholar
- Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129(3):775s–8. PubMedCASGoogle Scholar
- Zha S, Yin Y, Wang Y, Huang Y, Li Y, Wang Z. Cloning and functional analysis of farnesyl pyrophosphate synthase (FPPS) gene from Mylabris cichorii. Biotechnol Appl Chem. 2017;64(5):667–76. CASGoogle Scholar
- Fitton A, McTavish D. Pamidronate. A review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs. 1991;41(2):289–318. PubMedCASGoogle Scholar
- Xing X, Lian S, Hu Y, Li Z, Zhang L, Wen X, Du H, Jia Y, Zheng Z, Meng L, et al. Phosphatase of regenerating liver-3 (PRL-3) is associated with metastasis and poor prognosis in gastric carcinoma. J Translational Med. 2013;11:309. Google Scholar
- Bugni JM, Meira LB, Samson LD. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene. 2009;28(5):734–41. PubMedCASGoogle Scholar
- Song S, Yu Y. Progression on citrullination of proteins in gastrointestinal cancers. Front Oncol. 2019;9:15. PubMedPubMed CentralGoogle Scholar
- Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun rev. 2015;14(6):490–7. PubMedCASGoogle Scholar
- Tilvawala R, Nguyen SH, Maurais AJ, Nemmara VV, Nagar M, Salinger AJ, Nagpal S, Weerapana E, Thompson PR. The Rheumatoid Arthritis-Associated Citrullinome. Cell Chem Biology. 2018;25(6):691–704e696. CASGoogle Scholar
- Suzuki A, Yamada R, Yamamoto K. Citrullination by peptidylarginine deiminase in rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:323–39. PubMedCASGoogle Scholar
- Qu Y, Olsen JR, Yuan X, Cheng PF, Levesque MP, Brokstad KA, Hoffman PS, Oyan AM, Zhang W, Kalland KH, et al. Small molecule promotes β-catenin citrullination and inhibits wnt signaling in cancer. Nat Chem Biol. 2018;14(1):94–101. PubMedCASGoogle Scholar
- Funayama R, Taniguchi H, Mizuma M, Fujishima F, Kobayashi M, Ohnuma S, Unno M, Nakayama K. Protein-arginine deiminase 2 suppresses proliferation of colon cancer cells through protein citrullination. Cancer Sci. 2017;108(4):713–8. PubMedPubMed CentralCASGoogle Scholar
- Guo Q, Fast W. Citrullination of inhibitor of growth 4 (ING4) by peptidylarginine deminase 4 (PAD4) disrupts the interaction between ING4 and p53. J Biol Chem. 2011;286(19):17069–78. PubMedPubMed CentralCASGoogle Scholar
- Luo X, Chang S, Xiao S, Peng Y, Gao Y, Hu F, Liang J, Xu Y, Du K, Chen Y, et al. PAD4-dependent citrullination of nuclear translocation of GSK3β promotes colorectal cancer progression via the degradation of nuclear CDKN1A. Neoplasia (New York NY). 2022;33:100835. CASGoogle Scholar
- Yuzhalin AE, Gordon-Weeks AN, Tognoli ML, Jones K, Markelc B, Konietzny R, Fischer R, Muth A, O’Neill E, Thompson PR, et al. Colorectal cancer liver metastatic growth depends on PAD4-driven citrullination of the extracellular matrix. Nat Commun. 2018;9(1):4783. PubMedPubMed CentralCASGoogle Scholar
- Zheng Y, Zhao G, Xu B, Liu C, Li C, Zhang X, Chang X. PADI4 has genetic susceptibility to gastric carcinoma and upregulates CXCR2, KRT14 and TNF-α expression levels. Oncotarget. 2016;7(38):62159–76. PubMedPubMed CentralGoogle Scholar
- Chang XT, Wu H, Li HL, Li HL, Zheng YB. PADI4 promotes epithelial-mesenchymal transition(EMT) in gastric cancer via the upregulation of interleukin 8. BMC Gastroenterol. 2022;22(1):25. PubMedPubMed CentralCASGoogle Scholar
- Hao Y, Yu Y, Wang L, Yan M, Ji J, Qu Y, Zhang J, Liu B, Zhu Z. IPO-38 is identified as a novel serum biomarker of gastric cancer based on clinical proteomics technology. J Proteome Res. 2008;7(9):3668–77. PubMedCASGoogle Scholar
- Song S, Xiang Z, Li J, Ji J, Yan R, Zhu Z, Yu Y. A Novel Citrullinated modification of histone 3 and its Regulatory Mechanisms related to IPO-38 antibody-labeled protein. Front Oncol. 2019;9:304. PubMedPubMed CentralGoogle Scholar
- Mondal S, Thompson PR. Protein arginine deiminases (PADs): Biochemistry and Chemical Biology of protein citrullination. Acc Chem Res. 2019;52(3):818–32. PubMedPubMed CentralCASGoogle Scholar
- Lewis HD, Nacht M. iPAD or PADi-‘tablets’ with therapeutic disease potential? Curr Opin Chem Biol. 2016;33:169–78. PubMedCASGoogle Scholar
- Witalison EE, Cui X, Causey CP, Thompson PR, Hofseth LJ. Molecular targeting of protein arginine deiminases to suppress colitis and prevent colon cancer. Oncotarget. 2015;6(34):36053–62. PubMedPubMed CentralGoogle Scholar
- Ordóñez A, Yélamos J, Pedersen S, Miñano A, Conesa-Zamora P, Kristensen SR, Stender MT, Thorlacius-Ussing O, Martínez-Martínez I, Vicente V, et al. Increased levels of citrullinated antithrombin in plasma of patients with rheumatoid arthritis and colorectal adenocarcinoma determined by a newly developed ELISA using a specific monoclonal antibody. Thromb Haemost. 2010;104(6):1143–9. PubMedGoogle Scholar
- Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7(1):58–63. PubMedCASGoogle Scholar
- Kurmi K, Hitosugi S, Wiese EK, Boakye-Agyeman F, Gonsalves WI, Lou Z, Karnitz LM, Goetz MP, Hitosugi T. Carnitine palmitoyltransferase 1A has a lysine succinyltransferase activity. Cell Rep. 2018;22(6):1365–73. PubMedPubMed CentralCASGoogle Scholar
- Yang Y, Gibson GE. Succinylation Links metabolism to protein functions. Neurochem Res. 2019;44(10):2346–59. PubMedPubMed CentralCASGoogle Scholar
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Sci (New York NY). 2011;334(6057):806–9. CASGoogle Scholar
- Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, Chen W, Zhang S, Weiss RS, Locasale JW, et al. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc Natl Acad Sci USA. 2016;113(16):4320–5. PubMedPubMed CentralCASGoogle Scholar
- Weinert BT, Schölz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, Choudhary C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4(4):842–51. PubMedCASGoogle Scholar
- Mu R, Ma Z, Lu C, Wang H, Cheng X, Tuo B, Fan Y, Liu X, Li T. Role of succinylation modification in thyroid cancer and breast cancer. Am J cancer Res. 2021;11(10):4683–99. PubMedPubMed CentralCASGoogle Scholar
- Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflug Arch: Eur J Physiol. 2008;455(4):575–82. CASGoogle Scholar
- Wang C, Zhang C, Li X, Shen J, Xu Y, Shi H, Mu X, Pan J, Zhao T, Li M, et al. CPT1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J Cell Mol Med. 2019;23(1):293–305. PubMedCASGoogle Scholar
- Song Y, Wang J, Cheng Z, Gao P, Sun J, Chen X, Chen C, Wang Y, Wang Z. Quantitative global proteome and lysine succinylome analyses provide insights into metabolic regulation and lymph node metastasis in gastric cancer. Sci Rep. 2017;7:42053. PubMedPubMed CentralCASGoogle Scholar
- Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updates: Reviews Commentaries Antimicrob Anticancer Chemother. 2018;38:1–11. Google Scholar
- Zhu D, Hou L, Hu B, Zhao H, Sun J, Wang J, Meng X. Crosstalk among proteome, acetylome and succinylome in colon cancer HCT116 cell treated with sodium dichloroacetate. Sci Rep. 2016;6:37478. PubMedPubMed CentralCASGoogle Scholar
- Ren M, Yang X, Bie J, Wang Z, Liu M, Li Y, Shao G, Luo J. Citrate synthase desuccinylation by SIRT5 promotes colon cancer cell proliferation and migration. Biol Chem. 2020;401(9):1031–9. PubMedCASGoogle Scholar
- Qi H, Ning X, Yu C, Ji X, Jin Y, McNutt MA, Yin Y. Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress. Cell Death Dis. 2019;10(3):170. PubMedPubMed CentralGoogle Scholar
- Mead TJ, Martin DR, Wang LW, Cain SA, Gulec C, Cahill E, Mauch J, Reinhardt D, Lo C, Baldock C et al. Proteolysis of fibrillin-2 microfibrils is essential for normal skeletal development. eLife 2022, 11.
- Wang X, Shi X, Lu H, Zhang C, Li X, Zhang T, Shen J, Wen J. Succinylation inhibits the enzymatic hydrolysis of the Extracellular matrix protein fibrillin 1 and promotes gastric Cancer Progression. Advanced science (Weinheim. Baden-Wurttemberg Germany). 2022;9(27):e2200546. Google Scholar
- Ždralević M, Brand A, Di Ianni L, Dettmer K, Reinders J, Singer K, Peter K, Schnell A, Bruss C, Decking SM, et al. Double genetic disruption of lactate dehydrogenases a and B is required to ablate the Warburg effect restricting tumor growth to oxidative metabolism. J Biol Chem. 2018;293(41):15947–61. PubMedPubMed CentralGoogle Scholar
- Qanbar R, Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol Ther. 2003;97(1):1–33. PubMedCASGoogle Scholar
- Lum L, Clevers H. Cell biology. The unusual case of Porcupine. Sci (New York NY). 2012;337(6097):922–3. CASGoogle Scholar
- Schwab RHM, Amin N, Flanagan DJ, Johanson TM, Phesse TJ, Vincan E. Wnt is necessary for mesenchymal to epithelial transition in colorectal cancer cells. Dev Dynamics: Official Publication Am Association Anatomists. 2018;247(3):521–30. CASGoogle Scholar
- Wu GS. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009;285(1):1–5. PubMedCASGoogle Scholar
- Greenlee JD, Lopez-Cavestany M, Ortiz-Otero N, Liu K, Subramanian T, Cagir B, King MR. Oxaliplatin resistance in colorectal cancer enhances TRAIL sensitivity via death receptor 4 upregulation and lipid raft localization. eLife 2021, 10.
- Ascenzi P, Bocedi A, Marino M. Structure-function relationship of estrogen receptor alpha and beta: impact on human health. Mol Aspects Med. 2006;27(4):299–402. PubMedCASGoogle Scholar
- Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocrine-related Cancer. 2007;14(1):153–67. PubMedCASGoogle Scholar
- Caiazza F, Galluzzo P, Lorenzetti S, Marino M. 17Beta-estradiol induces ERbeta up-regulation via p38/MAPK activation in colon cancer cells. Biochem Biophys Res Commun. 2007;359(1):102–7. PubMedCASGoogle Scholar
- Ritter G, Cohen LS, Nice EC, Catimel B, Burgess AW, Moritz RL, Ji H, Heath JK, White SJ, Welt S, et al. Characterization of posttranslational modifications of human A33 antigen, a novel palmitoylated surface glycoprotein of human gastrointestinal epithelium. Biochem Biophys Res Commun. 1997;236(3):682–6. PubMedCASGoogle Scholar
- Lawson KA, Sousa CM, Zhang X, Kim E, Akthar R, Caumanns JJ, Yao Y, Mikolajewicz N, Ross C, Brown KR, et al. Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature. 2020;586(7827):120–6. PubMedPubMed CentralCASGoogle Scholar
- Du W, Frankel TL, Green M, Zou W. IFNγ signaling integrity in colorectal cancer immunity and immunotherapy. Cell Mol Immunol. 2022;19(1):23–32. PubMedCASGoogle Scholar
- Du W, Hua F, Li X, Zhang J, Li S, Wang W, Zhou J, Wang W, Liao P, Yan Y, et al. Loss of Optineurin Drives Cancer Immune Evasion via Palmitoylation-Dependent IFNGR1 lysosomal sorting and degradation. Cancer Discov. 2021;11(7):1826–43. PubMedPubMed CentralCASGoogle Scholar
- Yao H, Lan J, Li C, Shi H, Brosseau JP, Wang H, Lu H, Fang C, Zhang Y, Liang L, et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat Biomedical Eng. 2019;3(4):306–17. CASGoogle Scholar
- Duarte D, Nunes M, Ricardo S, Vale N. Combination of antimalarial and CNS drugs with Antineoplastic Agents in MCF-7 breast and HT-29 Colon cancer cells: Biosafety evaluation and mechanism of action. Biomolecules 2022, 12(10).
- Stamler JS, Lamas S, Fang FC. Nitrosylation. The prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–83. PubMedCASGoogle Scholar
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101(12):4003–8. PubMedPubMed CentralCASGoogle Scholar
- Hutchens S, Manevich Y, He L, Tew KD, Townsend DM. Cellular resistance to a nitric oxide releasing glutathione S-transferase P-activated prodrug, PABA/NO. Investig New Drugs. 2011;29(5):719–29. CASGoogle Scholar
- Nath N, Kashfi K, Chen J, Rigas B. Nitric oxide-donating aspirin inhibits beta-catenin/T cell factor (TCF) signaling in SW480 colon cancer cells by disrupting the nuclear beta-catenin-TCF association. Proc Natl Acad Sci USA. 2003;100(22):12584–9. PubMedPubMed CentralCASGoogle Scholar
- Williams JL, Ji P, Ouyang N, Kopelovich L, Rigas B. Protein nitration and nitrosylation by NO-donating aspirin in colon cancer cells: relevance to its mechanism of action. Exp Cell Res. 2011;317(10):1359–67. PubMedPubMed CentralCASGoogle Scholar
- Prévotat L, Filomenko R, Solary E, Jeannin JF, Bettaieb A. Nitric oxide-induced down-regulation of beta-catenin in colon cancer cells by a proteasome-independent specific pathway. Gastroenterology. 2006;131(4):1142–52. PubMedGoogle Scholar
- Gochman E, Mahajna J, Shenzer P, Dahan A, Blatt A, Elyakim R, Reznick AZ. The expression of iNOS and nitrotyrosine in colitis and colon cancer in humans. Acta Histochem. 2012;114(8):827–35. PubMedCASGoogle Scholar
- Gochman E, Mahajna J, Reznick AZ. NF-κB activation by peroxynitrite through IκBα-dependent phosphorylation versus nitration in colon cancer cells. Anticancer Res. 2011;31(5):1607–17. PubMedCASGoogle Scholar
- Ai Y, Kang F, Huang Z, Xue X, Lai Y, Peng S, Tian J, Zhang Y. Synthesis of CDDO-amino acid-nitric oxide donor trihybrids as potential antitumor agents against both drug-sensitive and drug-resistant colon cancer. J Med Chem. 2015;58(5):2452–64. PubMedCASGoogle Scholar
- Huang X, Dixit VM. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res. 2016;26(4):484–98. PubMedPubMed CentralCASGoogle Scholar
- Elguero B, Gonilski Pacin D, Cárdenas Figueroa C, Fuertes M, Arzt E. Modifications in the cellular proteome and their clinical application. Medicina. 2019;79(Spec 6/1):570–5. PubMedCASGoogle Scholar
- Yuan M, Song ZH, Ying MD, Zhu H, He QJ, Yang B, Cao J. N-myristoylation: from cell biology to translational medicine. Acta Pharmacol Sin. 2020;41(8):1005–15. PubMedPubMed CentralCASGoogle Scholar
- Pan S, Chen R. Pathological implication of protein post-translational modifications in cancer. Mol Aspects Med. 2022;86:101097. PubMedPubMed CentralCASGoogle Scholar
- Cox AD, Der CJ, Philips MR. Targeting RAS membrane Association: back to the future for Anti-RAS Drug Discovery? Clin cancer Research: Official J Am Association Cancer Res. 2015;21(8):1819–27. CASGoogle Scholar
- Prendergast GC, Rane N. Farnesyltransferase inhibitors: mechanism and applications. Expert Opin Investig Drugs. 2001;10(12):2105–16. PubMedCASGoogle Scholar
- Yang WS, Yeo SG, Yang S, Kim KH, Yoo BC, Cho JY. Isoprenyl carboxyl methyltransferase inhibitors: a brief review including recent patents. Amino Acids. 2017;49(9):1469–85. PubMedPubMed CentralCASGoogle Scholar
- Zhu D, Zhang Y, Wang S. Histone citrullination: a new target for tumors. Mol Cancer. 2021;20(1):90. PubMedPubMed CentralCASGoogle Scholar
- Zhu D, Lu Y, Wang Y, Wang Y. PAD4 and its inhibitors in Cancer Progression and Prognosis. Pharmaceutics 2022, 14(11).
Acknowledgements
This work was supported by the National Natural Science Foundation (82102740).




